Issues and updates related to the South Korean market. Notifications from the MFDS, market trends, public consultations, standards updates
Responsible Authorities in Korea FDA Medical devices in South Korea are managed by Medical Device Act and controlled by The Ministry of Food & Drug Safety (MFDS) or formerly Korea FDA. Under the jurisdiction of the Act, requirements for medical devices are provided in numerous various titles of guidelines. Below is a list of the…
South Korea regulation changes in 2018 The following regulatory changes are expected in 2018 in the South Korea regulation as published by the MFDS (Ministry of Food and Drug Safety): Main regulatory changes in South Korea regulation Adopt customized licensing system for advanced medical devices – ‘Special Act on Promotion of Advanced Medical Device Development…
Korea becomes the 10th member of IMDRF After the U.S., EU, Canada, Australia, Japan, China, Brazil, Russia, and Singapore, Korea has become an official member of the International Medical Device Regulatory Forum (IMDRF). This was announced officially on the Korea MFDS (Ministry of Food and Drug Safety) website as of December 11, 2017 and it…
Innovative medical devices in Korea – forecast analysis report The Korean MFDS published a forecast analysis report for innovative medical devices in South Korea. The link to the report is here. To help the medical device companies, Korean MFDS prepared and distributed the reports on the status and prospects of innovative medical devices(medical devices manufactured with…
KGMP guideline revision The Korean GMP (KGMP) guideline has been updated earlier this year (2016) and even if it was only provided in Korean we prepared a translated version in English. More details were provided in the guideline and the case of in vitro diagnostic equipment was considered since those were reclassified as medical devices.…
2016 is here now so Happy New Year first of all ! So in Korea now we are all one year older. Yes, A baby in Korea will not gain one year on his or her birthday. Instead, people will gain one year together on January 1st of every year (New Year’s Day). This is what “Korean age” is.…
This guideline for pulse oximeters was prepared to help the writing of the technical documentation and subsequent approval. pulse oximeters are commonly used to measure the concentration of oxygen in the blood. Main content of this guideline includes: medical equipment approval authorization process approval and technical requirement items, and examples of technical requirements. MFDS Evaluation…
Korean Ministry of Food and Drug Safety MFDS has announced that in order to expand European market of medical devices they are holding a working meeting at the MFDS office with Polish and Czech pharmaceutical and medical equipment registration office. This working meeting was arranged to follow up with high level officer meeting in 2013…
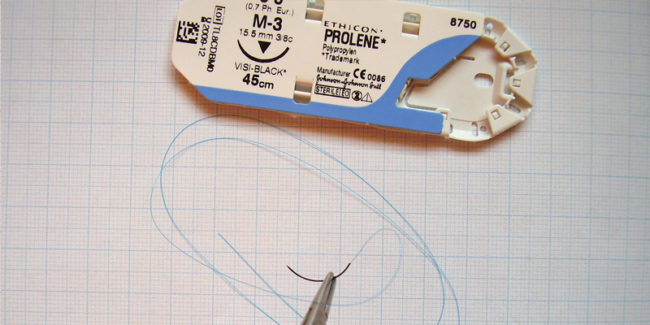
This guideline for polypropylene suture was prepared to help the writing of the technical documentation and subsequent approval. Polypropylene suture is a non-absorbable type of suture used for approximating and ligating soft tissue. Main content of this guideline includes: medical equipment approval authorization process approval and technical requirement items, and examples of technical requirements. MFDS…
In some cases performance requirements are not defined in the Korean regulation. That’s sometimes a good thing, and sometimes not. Performance test in Korea, means confirmation of the intended use and functional test at the same time. Let’s say for instance in the case of active devices, compliance with a particular standard like IEC 60601-2-X…