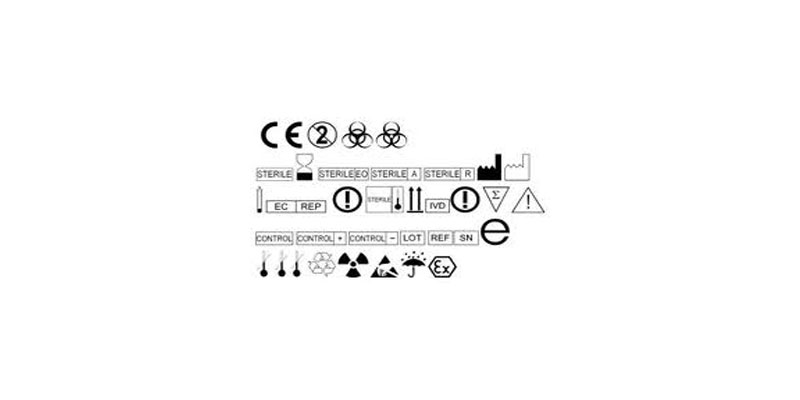
Manufacturers of medical devices in Europe won’t need to change their labeling.
Initially EN 980:2008 was about to be replaced by EN ISO 15223-1:2012 but as stated by the European Commission that won’t be the case. Some people were surprised seeing that no standard related to symbols was addressed anymore in the list of harmonized standards.
EN ISO 15223-1:2012 being qualified as deficient, the EN 980:2008 is likely to go back in the list.
Wait and see is sometimes the best thing to do…
If you wish to sell in South Korea you can contact us for a preliminary discussion. See also our services for the registration of medical devices in South Korea.