Issues and updates related to the South Korean market. Notifications from the MFDS, market trends, public consultations, standards updates
Ministry of Food and Drug Safety Notice No. 2014-155 was published on MFDS website. The Korean Good Manufacturing Practice (KGMP) being revised and this is a public consultation. South Korea medical device registration will be affected by this publication. The purpose here is clearly to improve the way KGMP is managed. Below is a summary…
Korean Ministry of Food and Drug Safety (MFDS) recently released a revision of its reference standard as per Ministry of Food and Drug Safety Notice No. 2014-122 affecting South Korea medical device registration. The purpose is to align with international standards related to electrical and mechanical safety. Are covered the following: radiation safety, usability, alarm…
Which one comes first among: KGMP (Korean Good Manufacturing Practice), product license, TDR (Technical Document Review) ? This is not a quiz, however often people are puzzled as to which one to apply for first. So far, the sequence has been like this: TDR product license KGMP The reason was that you needed the application…
A public consultation was published regarding the South Korea medical device regulations as below (Ministry of Food and Drug Safety Notice No. 2014-88 as of April 18, 2014) original notice is here: 1. Reasons for Revision A. Exempting license/notification of medical device for emergency such as life-threatening situations B. Clarifying the range of restriction…
A notice is given for Limited Revision regarding Medical Device Act 1081; released May 9, 2014 of the South Korea medical device regulations as below: 1. Reasons for Revision A. Subcontracting B. In-Vitro Diagnostic C.License Modification exemption D.Distribution Quality Management Standard E.Periodic Reporting F. Recall Notification The pharmaceutical drugs and the medical devices are currently…
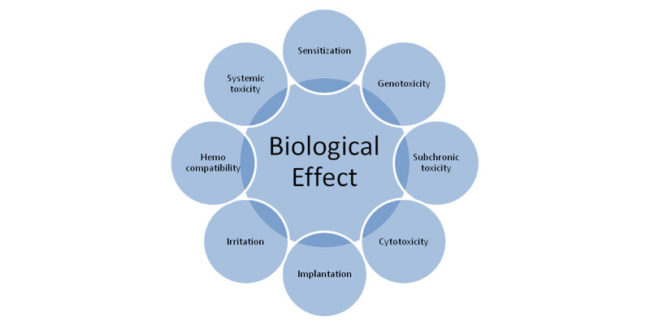
Limited Revision Notice regarding Biological Safety of Medical Devices published by South Korea MFDS Reasons for Revision (biological safety medical devices) The international requirements regarding the biological safety for medical devices that are internationally accepted has been partially changed subsequent to the revision of International Standards for medical devices (ISO 10993-4, 9, 10, 13, 16).…
Here is the Ministry of Food and Drug Safety Notice No. 2014-87 from South Korea. South Korea MFDS is revising 「Regulations regarding Medical Device Products and Product Classification」 (Ministry of Food and Drug Safety Notice No. 2014-88, 2/12/2014) in part, and gathering consensus from the people. They are hereby giving a notice regarding the purpose,…
As per Notice from MFDS: The Heart Rate Monitor and Pulse Monitor that are used for exercise and leisure purpose need not be included in the product classification for the medical purpose. In defining the Heart Rate Monitor and Pulse Monitor, those that are designed for exercise and leisure will be excluded (Appendix A26080.01, A26080.02).…
Finally available. Our translation of the Korean GMP Revision 2 in English is ready. The document was validated and is now ready for review. The main modifications are summarized below: Removed GMP inspection for class 1, but tightened for class 2, in the past, for class 2 only document inspection required, but since Jan 2014 it has been changed to site…
On April 2nd 2014, Ministry of Food and Drug Safety published on their website the second revision of their Korean GMP guideline. The analysis of the changes is currently being made and we will soon be able to provide a gap analysis as well as an English version of the document. Changes include for instance…