Biological safety Medical Devices in Korea
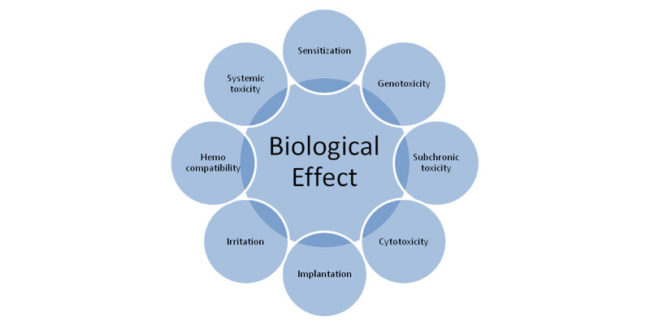
Limited Revision Notice regarding Biological Safety of Medical Devices published by South Korea MFDS Reasons for Revision (biological safety medical devices) The international requirements regarding the biological safety for medical devices that are internationally accepted has been partially changed subsequent to the revision of International Standards for medical devices (ISO 10993-4, 9, 10, 13, 16).…