Change that will not affect the status of registration of the product or changes not requiring a significant amount of work. Compliance may not be affected.
Reclassification and re-categorization of medical devices The Ministry of Food and Drug Safety (MFDS) is publishing Korea medical device regulations update on a regular basis. It recently published a notice to inform that a reclassification and re-categorization of products would occur. Reclassification of 244 products previously known as in vitro radio-pharmaceuticals to medical devices guideline…
The Notified Bodies Oversight Group (NBOG) has published (Nov. 2014) the following revised Notified Body guidance documents. The aim of such guidance is to address some of the best practice expected from manufacturers and Notified Bodies. The guidance documents can be found on the below link: 1. NBOG BPG 2014-3 – Guidance for manufacturers and…
Background Recently Korean Ministry of Food and Drug Safety (MFDS previously called KFDA) published its quality management standard for distributors operating in South Korea. Is your distributor ready ? After strengthening its regulation for license holders and require them to appoint a dedicated Quality Manager (effective since July 2014), MFDS is now regulating the distribution…
Notification of regulation amendment regarding approval, notification and evaluation of medical devices in Korea. A) Implantable Medical Device B) Restricted substances C) Intended Use D) Clinical Evaluation E) Medical apps Korean medical devices regulation update 2014-142 A) Implantable Medical Device The definition of Implantable medical device and its application (Article 2 and Article 5) 1)…
borderline and classification medical devices: UPDATED MANUAL ON BORDERLINE AND CLASSIFICATION IN THE COMMUNITY REGULATORY FRAMEWORK FOR MEDICAL DEVICES Version 1.16 (07-2014) Borderline cases are considered to be those cases where it is not clear from the outset whether a given product is a medical device, an in vitro diagnostic medical device, an active implantable…
Which one comes first among: KGMP (Korean Good Manufacturing Practice), product license, TDR (Technical Document Review) ? This is not a quiz, however often people are puzzled as to which one to apply for first. So far, the sequence has been like this: TDR product license KGMP The reason was that you needed the application…
A public consultation was published regarding the South Korea medical device regulations as below (Ministry of Food and Drug Safety Notice No. 2014-88 as of April 18, 2014) original notice is here: 1. Reasons for Revision A. Exempting license/notification of medical device for emergency such as life-threatening situations B. Clarifying the range of restriction…
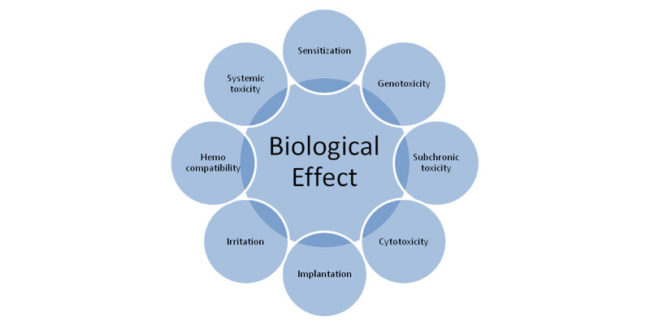
Limited Revision Notice regarding Biological Safety of Medical Devices published by South Korea MFDS Reasons for Revision (biological safety medical devices) The international requirements regarding the biological safety for medical devices that are internationally accepted has been partially changed subsequent to the revision of International Standards for medical devices (ISO 10993-4, 9, 10, 13, 16).…
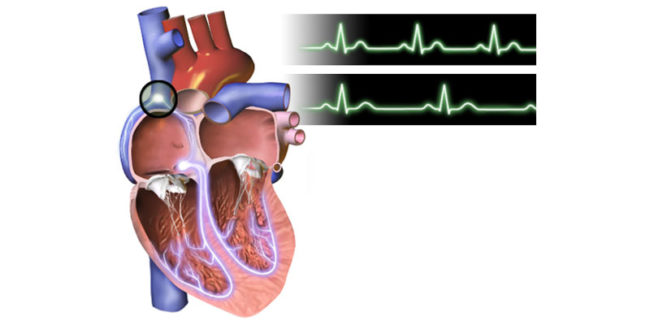
As per Notice from MFDS: The Heart Rate Monitor and Pulse Monitor that are used for exercise and leisure purpose need not be included in the product classification for the medical purpose. In defining the Heart Rate Monitor and Pulse Monitor, those that are designed for exercise and leisure will be excluded (Appendix A26080.01, A26080.02).…
In a very similar way as US FDA addressed this topic, South Korean MFDS has announced measures to distinguish mobile medical devices from more fitness related application or devices. The original public consultation is posted here. Comments from the public can be submitted until April 7, 2014. For instance, heart rate monitors and pulse rate…